Bangladesh approves Chinese vaccine CoronaVac for emergency use
Published:
2021-06-06 18:18:00 BdST
Update:
2026-02-05 02:36:39 BdST
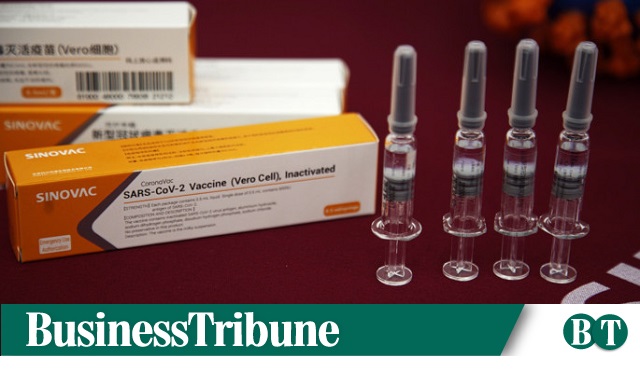
Directorate General of Drug Administration (DGDA) has proved the Chinese vaccine CoronaVac, made by Sicovac life Sciences Co.
Incepta Vaccine Ltd had applied to the DGDA for the approval of the vaccine on 3 June.
In a press release today, the DGDA said that CoronaVac is the fifth vaccine to be given the Emergency Use Authorization (EUA).
This vaccine has received the EUA in 22 other countries. World Health Organization included the vaccine in the Emergency Use Listing on 1 June.
The approved vaccines that are currently being administered in Bangladesh:
COVISHIELD, also known as SARS-CoV2 AZD1222, Oxford/Astra Zeneca vaccine, made by M/S Serum Institute of India Pvt, India which was given EUA on 7 January.
Sputnik V (Gam-Covid-Vac), made by GENERIUM Joint Stock Company, Russia, which received EUA on 27 April.
Zhong Al Ke Wei COVID- 19 Vaccine (vero cell), Inactivated (Sinopharm) made by Beijing Institute of Biological Products Co. Ltd, China, which received EUA on 29 April.
Tozinameran-COVID-19 mRNA vaccine (nucleoside modified)/BTN I 62b2 (Trade Name: COMIRNATY), made by Pfizer Manufacturing Belgium NV, Belgium which was given EUA on 27 May.
Topic:

Deputy Commissioner of Dhaka won Digital Bangladesh Award 2020

Government has punished Mahbub Kabir Milon

COVID-19 infections cross 2.50 lakh mark in Bangladesh

'OSD' Additional Secretary of Railways Mahbub Kabir Milon

Dhamaka sent customer's advance money to US!


Leave your comment here: